画像 homogeneous mixture vs heterogeneous mixture chemistry 994612-Homogeneous mixture vs heterogeneous mixture chemistry
The elements and substances used in homogeneous mixtures are not easily dissolved 3 The term 'homo' means identical The term 'hetero' means dissimilar 4 The texture formed of a homogeneous mixture is smooth The texture formed of a heterogeneous mixture is not smooth 5 The size of the particle in a homogeneous mixture is that of an atomLike us on Facebook https//wwwfacebookcom/k12mojoFollow us on twitter https//twittercom/K12MojoDownload our App https//playgooglecom/store/appMixtures formed by combining two or more materials either form homogeneous or heterogeneous mixturesHomogeneous mixture comes from homo (th

Homogeneous Mixture Definition Examples Tutors Com
Homogeneous mixture vs heterogeneous mixture chemistry
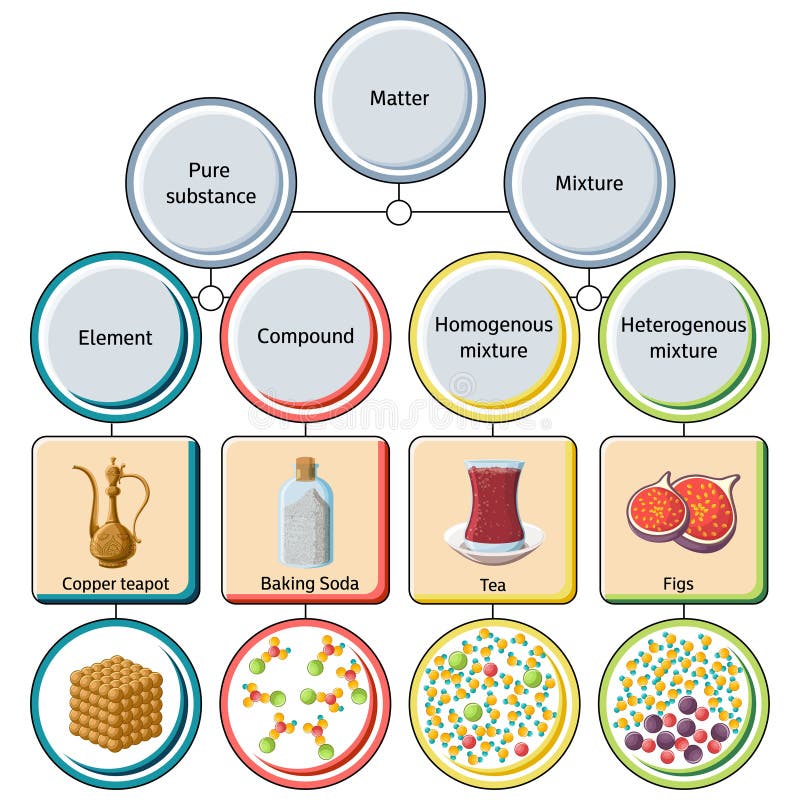
Homogeneous mixture vs heterogeneous mixture chemistry- Mixtures can be mainly divided into two as homogeneous mixtures and heterogeneous mixtures A heterogeneous mixture has two or more phases and the components can be individually identified A homogeneous mixture is uniform;Homogeneous Mixture Vs Heterogeneous Mixture The comparison between heterogeneous and homogenous mixtures presented here, will clear all doubts regarding the differences between these two types These are two of most fundamental concepts in chemistry




Heterogeneous And Homogeneous Mixtures Write And Draw Worksheet Teaching Chemistry Middle School Science Experiments Middle School Science Resources
Answer choices heterogeneous mixture solid mix s Question 15 SURVEY 60 seconds Q Italian Salad Dressing is a answer choices #Homogeneous #Heterogeneous #Mixture #Difference #homogeneous #heterogeneous #mixture chemistry,heterogeneous,homogeneous,homogeneous mixture example,heterogeneous mixture examples,homogeneous vs heterogeneous matter,types of heterogeneous mixture,homogeneous solution,homogeneous and heterogeneous mixtures,homogeneous mixtures,heterogeneousTherefore, the individual components cannot be separately identified
The mixture can either be homogenous or heterogeneous, depending on the constituents of the mixture Homogenous mixtures consist of two or more substances that are mixed in a way that the constituents cannot be observed by simple physical meansCheerios Trail mix Trail mix is HETEROGENEOUS Cheerios is homogeneousStart studying Chapter 1 Homogeneous VS Heterogeneous Mixture Learn vocabulary, terms, and more with flashcards, games, and other study tools
Start studying Heterogeneous vs Homogeneous Mixtures Learn vocabulary, terms, and more with flashcards, games, and other study tools The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed together and the uniformity of their composition A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture The composition of the mixture is the same throughout3 Make a table to compare and contrast solutions, colloids and suspensions Include an example of each type of mixture in your table 4 Iron filings are attracted by a magnet This is a physical property of iron but not of most other materials, including sand




Homogeneous Mixture Examples With Chemical Formula




Ppt Homogeneous And Heterogeneous Mixtures Powerpoint Presentation Free Download Id
Heterogeneous vs Homogeneous Mixtures How are they different?L1 Chemistry Mixtures Classifying Heterogeneous vs Homogeneous Mixturespptx 3 Download and print the Worksheet 31 When completed, please upload to MANAGEBAC 4 Create a VIDEO (and upload to SEESAW) discussing 3 different types of mixtures you can find in your kitchen You can do this as you make a meal, or simply find things in your fridge!Play this game to review Chemistry Freshly brewed black coffee Preview this quiz on Quizizz What is a homogeneous mixture?



Homogeneous And Heterogeneous Mixtures




Homogeneous And Heterogeneous Mixtures Examples Classification Of Matter Chemistry Youtube
Homogeneous mixtures exist in one phase of matter at a time You will not see liquid water and solid water together in a homogeneous mixture That means your glass of ice water, with ice cubes floating in it, is a heterogeneous mixture of homogeneous mixtures Homogeneous mixtures cannot be expressed as chemical formulasUniform appearance and composition throughout Many homogeneous mixtures are commonly referred to as solutions A heterogeneous mixture consists of visibly different substances or phases The three phases or states of matter are gas, liquid, Homogeneous Mixture vs Heterogeneous Mixture A mixture is the combination of two or more pure substance in such a way that they are not chemically united The each pure substance getting into make the mixture have an influence on the mixture as it shows on the type of properties For instance, with making a mixture of sugar and water, the




2 2 Mixtures Classification Of Matter Siyavula



1
Summary A mixture is a physical blend of two or more components, each of which retains its own identity and properties in the mixture A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture All solutions would be considered homogeneous A heterogeneous mixture is a mixture in which the composition is not uniform throughout theHomogeneous and heterogeneous mixtures In chemistry, if the volume of a homogeneous suspension is divided in half, the same amount of material is suspended in both halves of the substance An example of a homogeneous mixture is air In physical chemistry and materials science this refers to substances and mixtures which are in a single phaseThis is in contrast toStart studying Homogeneous mixture vs Heterogeneous mixture Learn vocabulary, terms, and more with flashcards, games, and other study tools



Homogeneous And Heterogeneous Mixture Nine Science




Heterogeneous And Homogeneous Mixtures Write And Draw Worksheet Teaching Chemistry Middle School Science Experiments Middle School Science Resources
A chemistry experiment demonstrates how to tell the difference between heterogeneous mixtures and homogeneous mixtures using a flashlight4 $395 $295 PDF This 3 page worksheet covers the topics of elements, compounds and mixtures, homogeneous vs heterogeneous mixtures, extensive vs intensive properties, physical and chemical properties, physical and chemical changes, and states of matter Information is presented in a logical progression and has e The difference between homogeneous mixtures and heterogeneous mixtures is a matter of scale the heterogeneous mixture can be seen on beaches where sand included many particles like coral, shells and organic matter, etc they all can be separated easily hence known as a heterogeneous mixture but when we take a large amount of sand, it's impossible to separate all the matter, which terns as a homogeneous mixture




Compound Vs Mixture Difference And Comparison Diffen




Homogenous And Heterogenous Mixtures Chemistry For Non Majors
A mixture consists of sugar in water is a homogeneous mixture because we can't see particles of sugar in the water, as they are dissolved thoroughly A mixture consisting of oil in water is an example of the heterogeneous mixture as the oil cannot be mixed in the water and we can easily see them I hope you like my post about "DifferenceAnswer choices a mixture that is the same throughout a mixture that is made up of one type of atom a mixture that is made up of one type of compound a mixture that cannot be separated through physical changes s Question 13 SURVEYChemistry Mixtures And Solutions Study Guide SOLUTIONS are homogeneous mixtures A solution is a mixture of two or more substances in a single phase Page 3/14 Read PDF Chemistry Mixtures And Solutions Study Guide At least two substances must be mixed in order to have a solution The Mixtures And Solutions Study Guide Chemistry



1
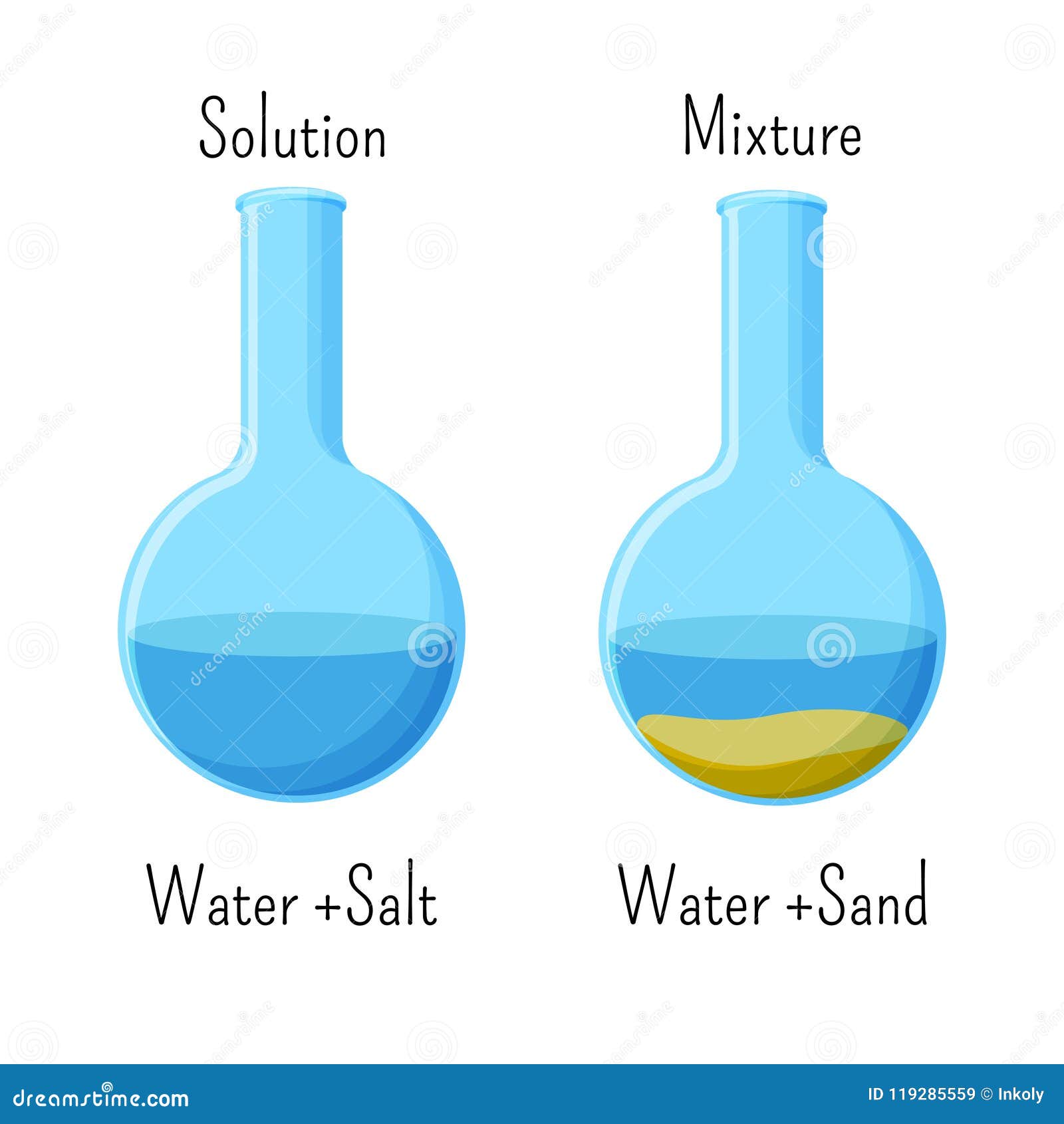



Homogeneous Solution Of Water And Salt And Heterogeneous Mixture Of Water And Sand In Glass Beakers Stock Illustration Illustration Of Icon Clipart
Homogeneous blends just have one stage gas, fluid or strong Different homogenous blends are air, water and vodka Heterogeneous Mixtures Heterogeneous blends are comprised of noticeably extraordinary substances or stages A suspension is a kind of heterogeneous blend with huge particles, obviousLearn heterogeneous mixture science chemistry with free interactive flashcards Choose from 500 different sets of heterogeneous mixture science chemistry flashcards on Quizlet If a substance is not chemically pure, it is either a heterogeneous mixture or a homogeneous mixture If its composition is uniform throughout, it is a homogeneous mixture Solution A) Tea is a solution of compounds in water, so it is not chemically pure It is usually separated from tea leaves by filtration




Homogeneous And Hetrogeneous Mixtures Definition Examples Teachoo
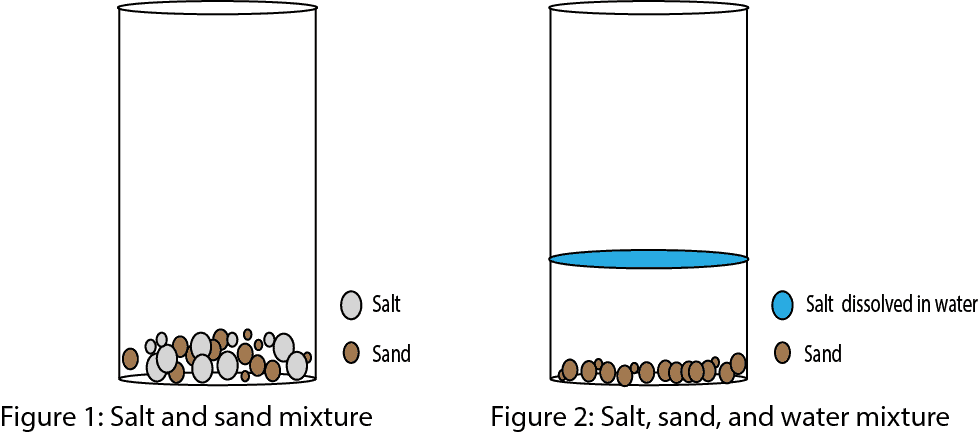



What S A Mixture How Does Heterogeneous Differ From Homogeneous Mixture And How Can Mixtures Be Separated
What is the difference between a homogeneous and a heterogeneous mixture? By definition, a pure substance or a homogeneous mixture consists of a single phase A heterogeneous mixture consists of two or more phases When oil and water are combined, they do not mix evenly, but instead form two separate layers Each of the layers is called a phase Oil and water do not mix, instead forming two distinct layers called phases Homogenous vs Heterogeneous Matter created by Simmy8 on 21 Jun 12, enabled by jimmy Sciences Medium level (75% of success) 15 questions 52 033 players Classify the following substances and mixtures as either homogeneous or heterogeneous 1




Let S Talk Chemistry All About Mixtures



1
Heterogeneous and Homogeneous Mixtures are discussed in this presentation High School chemistry, physical science, environmental science, earth systems, and Slideshare uses cookies to improve functionality and performance, and to provide you with relevant advertisingStart studying Homogeneous mixture vs Heterogeneous mixture Learn vocabulary, terms, and more with flashcards, games, and other study toolsChemistry Classifying Matter Name_____ Classify each of the materials below In the center column, state whether the material is a pure substance or a mixtureIf the material is a pure substance, further classify it as either an




What Is A Homogeneous Mixture Definition And Examples



Homogeneous And Heterogeneous Mixtures
Mixtures are combination of two or more substances where each substance retains its individual physical properties There are two classes of mixtures homogeneous and heterogeneous Homogeneous mixtures have a constant composition whereas heterogeneous mixtures do not Another name for a homogeneous mixture is a solutionQ Type of mixture that DOESN'T HAVE the same composition in every partActivity This one page worksheet will give your students practice with heterogeneous and homogeneous mixtures The topics of the questions include prefix meanings, examples, differences between the two types of mixtures, and the definition of mixtures The worksheet involves critical thinking skills and will




Homogeneous And Heterogeneous Mixtures Activities




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii
HOMOGENEOUS MIXTURE VS HETEROGENEOUS MIXTURE CLASSIFICATION OF MIXTURES TYPES OF MIXTURES CHEMISTRY Homogeneous Mixtures Homogeneous mixturesHomogeneous mixtures have uniform composition Heterogeneous mixtures have nonuniform composition What type of mixtures are these?This chemistry video tutorial explains the difference between homogeneous and heterogeneous mixtures within the subtopic of the classification of matter It



Is Sugar A Homogeneous Or Heterogeneous Mixture Chemistry Point
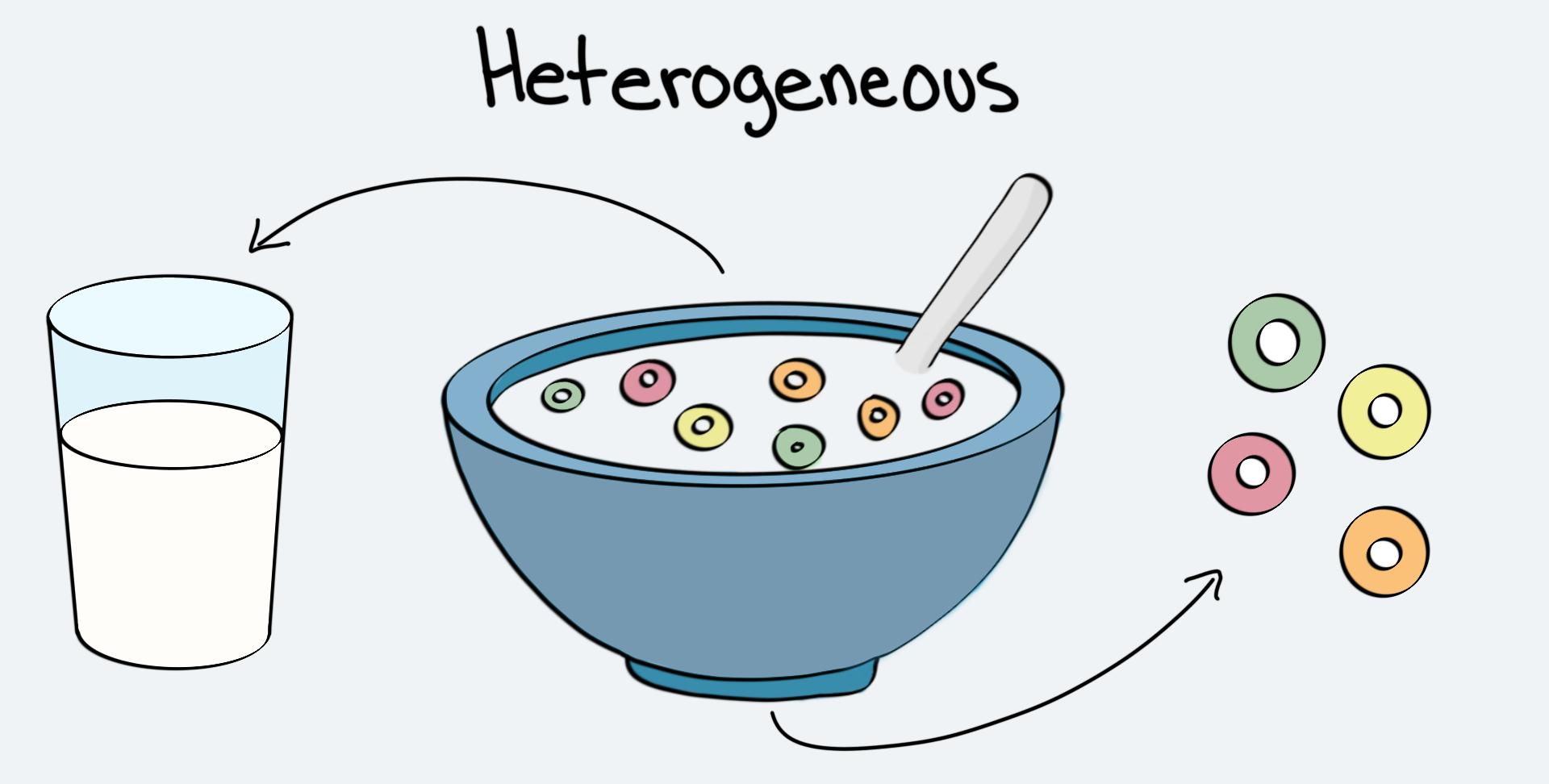



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii
By definition, a pure substance or a homogeneous mixture consists of a single phase A heterogeneous mixture consists of two or more phases When oil and water are combined, they do not mix evenly, but instead form two separate layers Each of the layers is called a phaseA homogeneous mixture has a uniform composition and appearance Individual substances that constitute a homogeneous mixture cannot be visually differentiated On the other hand, a heterogeneous mixture comprises two or more substances that can be distinctly observed, and even separated relatively easilyMixtures Homogeneous, Heterogeneous I First Impressions (≈ 1 minute) Ensures the line is silent, greet students at the door, shake their hand "Every day, this is how you will come in line up silently by the door, shake my hand, and find your seat silently These will be your assigned seats, unless otherwise told so




Homogeneous Mixture And Heterogeneous Mixture Is Matter Around Us Pure Chemistry Class 9 Youtube




What Do You Need To Know About Heterogeneous And Homogeneous Mixtures
A heterogeneous mixture is a type of mixture that allows the components to be seen as two or more phases are present A mixture is an example of water Water is a homogeneous mixture of nitrogen, oxygen and smaller amounts of other compounds in the gaseous materials Stay tuned with BYJU'S to learn more interesting topics in Chemistry The difference between a homogeneous mixture and a heterogeneous mixture is that, unlike heterogeneous mixtures, homogeneous mixtures are consistent, meaning their constitution is the same no matter where one looks In physical chemistry and materials science, the definition of a heterogeneous mixture is somewhat different Here, a homogeneous mixture is one in which all components are in a single phase, while a heterogeneous mixture contains components in different phases Examples of Heterogeneous Mixtures




Homogeneous Mixture Example Food




Homogeneous Mixture Examples In Kitchen
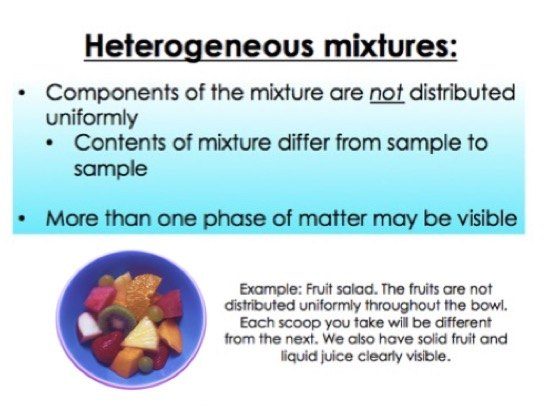
A heterogeneous mixture is the type of mixture in which the composition of the solute is not uniform throughout the mixture Thus, in a heterogeneous mixture, all parts of the mixture do not have the same concentration throughout The components of a heterogeneous mixture are easily visible in the mixture as the size of the particles tends to
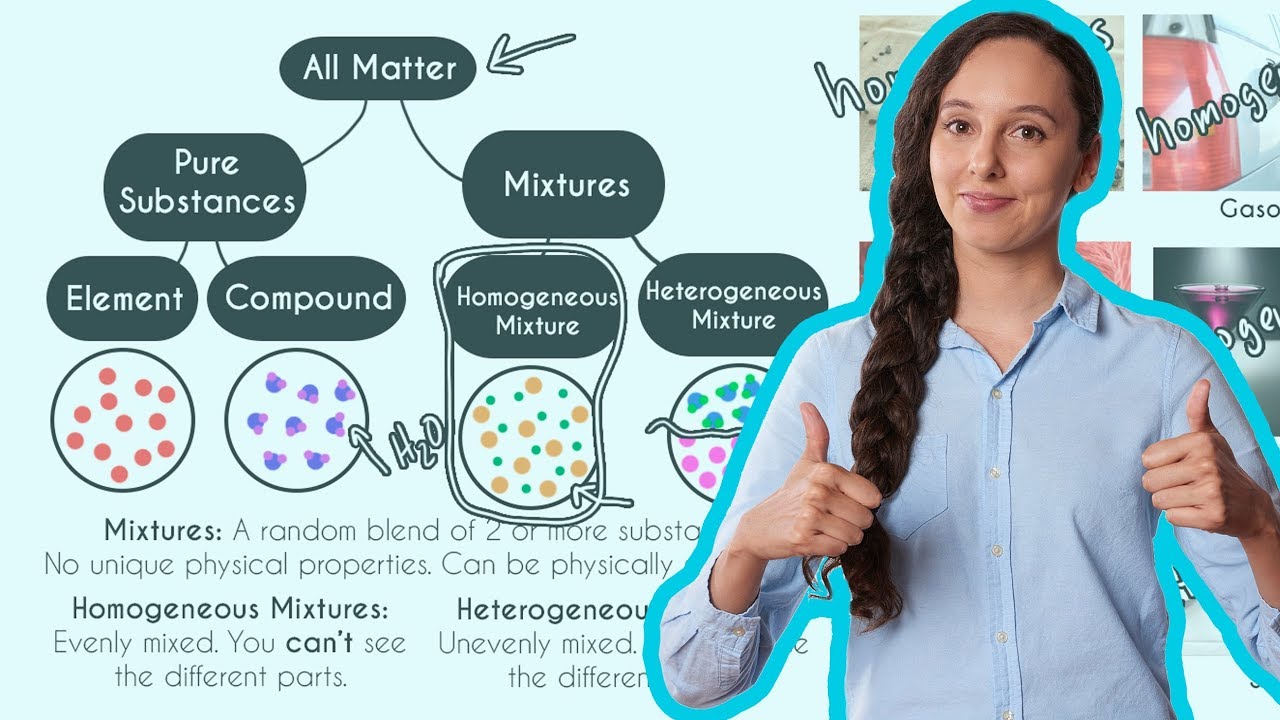



Homogeneous And Heterogeneous Mixtures Youtube




What Is The Difference Between A Homogeneous Mixture And A Heterogeneous Mixture




What S The Difference Between Heterogeneous And Homogeneous Mixtures Homogeneous Mixture Examples Of Mixtures Teaching Chemistry




10 Examples Of Mixtures



Mixture



3




Topic 2 Mixtures Activities
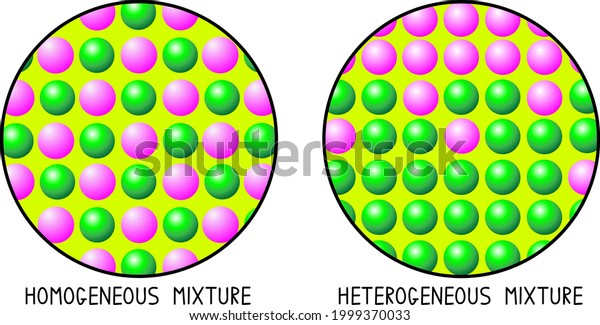



Homogeneous Mixture Vs Heterogeneous Mixture Particle Stock Vector Royalty Free



Homogeneous Mixture Stock Illustrations 7 Homogeneous Mixture Stock Illustrations Vectors Clipart Dreamstime



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples




How To Identify Heterogeneous Homogeneous Mixtures




Difference Between Homogeneous Mixture And Heterogeneous Mixture Youtube



Homogeneous And Heterogeneous Mixtures




How To Identify Heterogeneous Homogeneous Mixtures




What Is A Heterogeneous Mixture Definition And Examples




Homogeneous And Heterogeneous Mixtures




Homogeneous And Heterogeneous Mixture Difference Between Homogeneous And Heterogeneous Mixture Youtube




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




Heterogeneous Mixture And Homogeneous Mixture Youtube




List The Points Of Difference Between Homogeneous And Heterogeneous Mixtures Brainly In




2 2 Mixtures Classification Of Matter Siyavula




3 4 Classifying Matter According To Its Composition Chemistry Libretexts



Homogeneous And Heterogeneous Mixtures
/definition-of-heterogeneous-mixture-and-examples-605206_final23-ecfa4da6517640429448462eae1f09f7.png)



Definition Of Heterogeneous Mixture With Examples
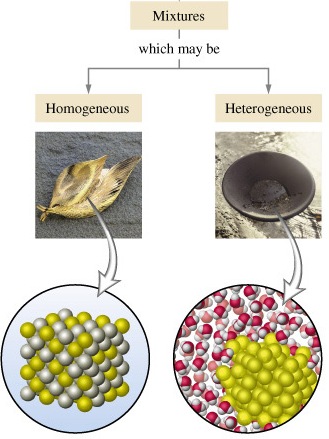



Difference Between Homogeneous Mixture And Heterogeneous Mixture




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




Homogeneous Mixture Definition Examples Tutors Com
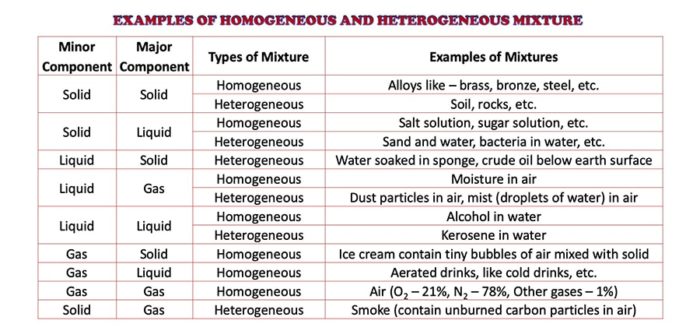



Homogeneous Heterogeneous Mixture Definition Examples Selftution




Homogeneous And Heterogeneous Mixtures Worksheet Middle School Science Challenging Critica Teaching Science Middle School Science Experiments Science Lessons
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



Heterogeneous Vs Homogeneous Mixtures



Mixture



Chapter 3 Chemistrysaaakollasch




The Homogeneous And Heterogeneous Mixture Diagram Quizlet




What Is The Difference Between A Homogeneous Mixture And A Heterogeneous Mixture
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



Heterogeneous Vs Homogeneous Mixtures




Homogenenous And Heterogeneous Mixtures Youtube




Chemistry




Heterogeneous And Homogeneous Mixture Differences Videos Examples




Mixtures And Solutions Cpd Rsc Education
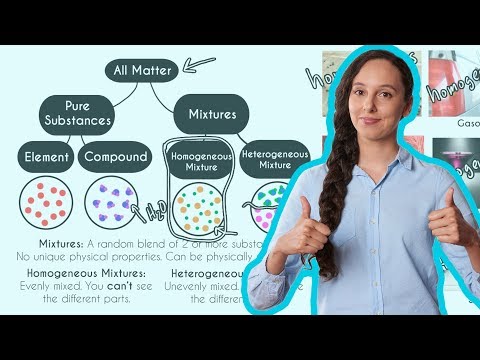



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples




Homogenous And Heterogenous Mixtures Chemistry For Non Majors




Shutterstock Puzzlepix




Mixture Wikiwand




Ch 12 1 Types Of Mixtures Ppt Video Online Download



Mixture




Homogenous And Heterogenous Mixtures Chemistry For Non Majors




Heterogeneous Vs Homogeneous Mixtures




What Is The Difference Between A Homogeneous Mixture And A Heterogeneous Mixture Bitwise Academy




Difference Between Homogeneous And Heterogeneous Homogeneous Vs Heterogeneous



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii



Is Chocolate Chip Ice Cream Homogeneous




Homogenous And Heterogenous Mixtures Chemistry For Non Majors




Homogenous Mixture Worksheets Teaching Resources Tpt




How To Identify Heterogeneous Homogeneous Mixtures




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




Heterogeneous And Homogeneous Mixtures Youtube
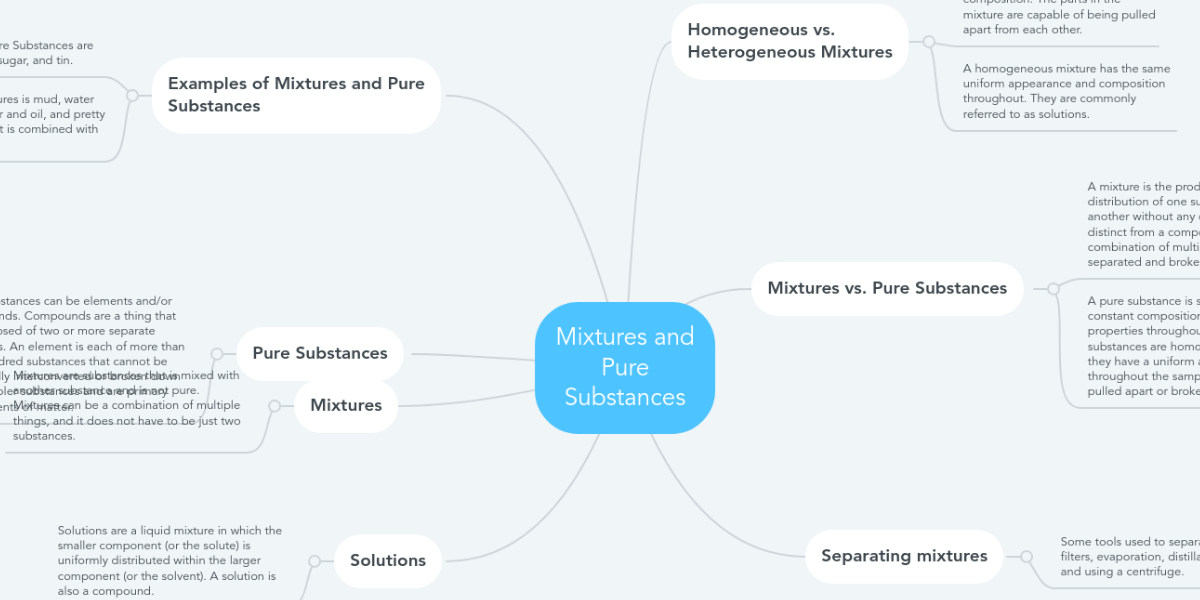



Mixtures And Pure Substances Mindmeister Mind Map



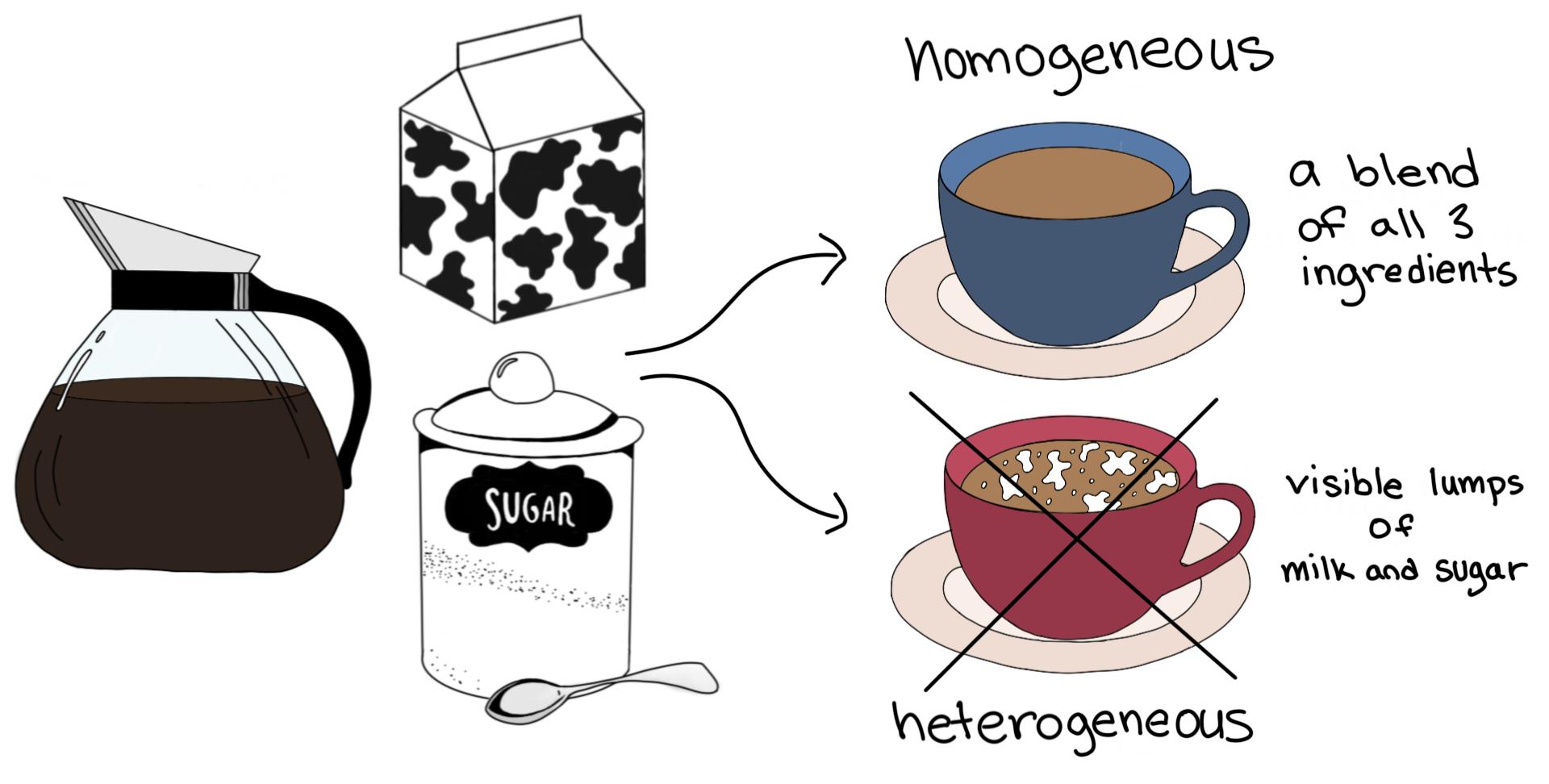
Homogeneous Vs Heterogeneous Mixtures A Comparison Expii
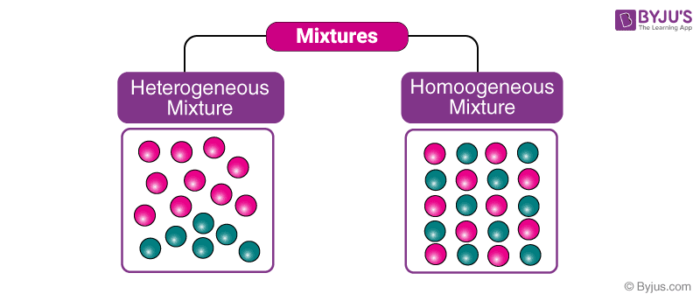



Heterogeneous And Homogeneous Mixture Differences Videos Examples




Homogeneous Heterogeneous Mixture Definition Examples Selftution




Homogeneous Vs Heterogeneous Mixtures Youtube




Homogeneous Mixture Vs Heterogeneous Mixture Classification Of Mixtures Types Of Mixtures Chemistry Youtube



Is Wood Homogeneous Or Heterogeneous Quora



Chemistry Quilchena Div Q 18 19



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples




How To Identify Heterogeneous Homogeneous Mixtures




Heterogeneous And Homogeneous Mixtures What S The Difference Homogeneous Mixture Heterogeneous Mixture Chemistry
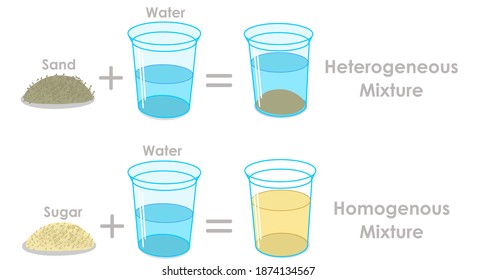



Mixtures Images Stock Photos Vectors Shutterstock




Homogeneous And Heterogeneous Mixtures Png Images Pngwing
:max_bytes(150000):strip_icc()/GettyImages-548326197-58fe30b63df78ca159cb3f67.jpg)



10 Heterogeneous And Homogeneous Mixtures




Examples Of Heterogeneous Mixtures Types Made Simple




What Is The Difference Between Heterogeneous Mixture Vs Homogenous Mixture Brainly Com




Substances And Mixtures Introduction To Chemistry




Mixtures Homogeneous And Heterogeneous Mixtures Ppt Video Online Download
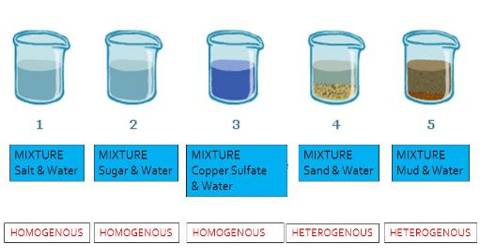



Homogeneous Mixture Experiment Qs Study




Homogeneous Mixture Examples In Daily Life




Heterogeneous Homogeneous Mixture Card Sort For Matter In Chemistry Homogeneous Mixture Heterogeneous Mixture Sorting Cards
コメント
コメントを投稿